The Mayne Pharma Group Ltd (ASX: MYX) share price crashed 17% on Tuesday on negative news received from the US Food and Drug Administration (FDA).
Mayne Pharma develops, manufactures and markets branded and generic pharmaceutical products globally.
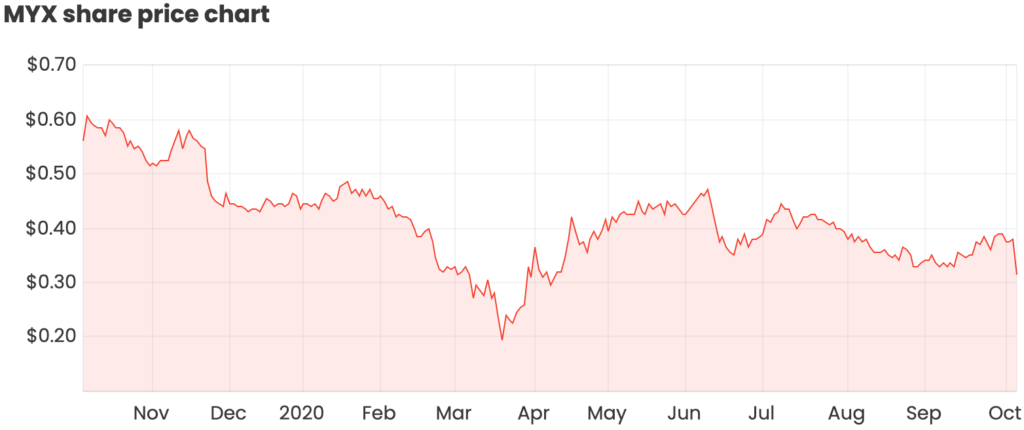
What caused investors to dump their MYX shares?
Yesterday, Mayne Pharma revealed it had received a complete response letter from the FDA regarding its application for a generic version of Nuvaring – one of the most popular contraceptive products sold in the US.
Mayne Pharma CEO Scott Richards said, “We are confident we can address the issues raised in the letter in a timely manner. Pleasingly, the FDA has indicated that Mayne Pharma and its development partner Mithra have an acceptable manufacturing process for generic NUVARING”.
Scott confirmed that if approved, the generic Nuvaring product has a total addressable market of US$920 million. Based on yesterday’s crash in the Mayne Pharma share price, it is clear investors were factoring in a high chance of FDA approval.
Mayne’s product portfolio
You would not know it from watching Mayne’s share price, but the generic Nuvaring product is not its only product.
In July 2016, Mayne Pharma paid US$652 million (AUD$911.5 million) for 42 generic drugs from Teva Pharmaceutical Industries. Of these, 37 products were approved by the FDA, with the remaining 5 pending approval.
The market will be closely tracking the company’s products pending FDA approval, so we could expect to see similar volatility in the Mayne share price going forward.
Are Mayne shares a buy?
After Tuesday’s share price collapse, Mayne Pharma has a market capitalisation of just $529 million. This is significantly less than what Mayne paid for the portfolio of generic drugs from Teva just 4 years ago.
In my view, if any of Mayne’s pipeline products gain FDA approval, today’s share price will look cheap. That said, I would like to gain a better understanding of Mayne’s products before buying in.





