Today, Avita Medical Inc (ASX: AVH) reported positive numbers for its second-quarter (Q2) of FY21 and the share price sank 11%. Is Avita on its way to restoring its share price to pre-COVID levels?
Avita is a regenerative medicine business with a technology platform positioned to address unmet medical needs in burn injuries, trauma injuries, chronic wounds, and other dermatological conditions. The patented technology provides treatment solutions using the properties of a patient’s own skin cells.
RECELL is the company’s flagship product. The product takes a small amount of the patient’s skin as a sample and in around 30 minutes a spray can be created and applied.
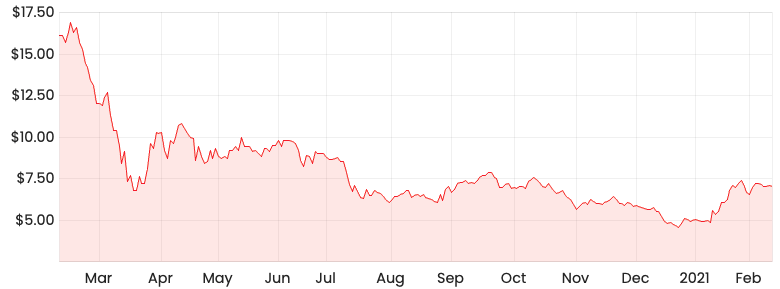
AVH share price
Q2 FY21 highlights
Avita posted US-based revenue of $5 million for its key product, RECELL, in this quarter — a 62% jump over the prior corresponding period, Q2 FY20 (PCP).
In terms of total global revenue, Avita generated $5.1 million, resulting in a 57% boost relative to the PCP.
The procedural volumes remained consistent at 487 for this quarter, a slight decrease from 496 in the prior quarter ended September FY20. However, AVITA continues to sign up new accounts, adding 7 new accounts in this quarter, taking the total to 93 accounts.
Avita noted more patients enrolled in its study to assess the use of the RECELL System to treat stable vitiligo. Vitiligo is a disease that causes the loss of skin colour in blotches and Avita believes this represents a significant market. In Avita’s FY20 annual report, it notes the estimated worldwide vitiligo prevalence is between 0.5-2% of the population.
The company also appears to be better at managing its costs as gross margins increased from 74% in the PCP to 84% in this quarter.
CEO outlook
Dr Mike Perry, Avita Medical Chief Executive Officer seems to be optimistic about Aita’s outlook, “I’m proud of our progress over the last quarter as we strive to broaden the applications of our platform to serve patients both within burns and beyond. With our burn centre account base now mostly built out, our sales team is poised and ready to drive utilization as the pandemic abates and we regain access to hospitals and patients. We have continued to make strong progress with our vitiligo pivotal trial, seeing very encouraging interest and enrollment trends, and we believe this could put us in a position to file for FDA approval in 2022″.
Avita’s future
Despite the significant drop in Avita’s share price since COVID-19, the business has managed to continue to grow its revenue and keep improving its cost management. No wonder Dr Perry appears upbeat about Avita’s prospects.
In saying that, Avita recorded its most significant net loss in FY20 due to heavy allocation towards commercialisation of the RECELL System. Investors should be mindful that Avita is still an early-stage growth company trying to grab market share, so selling and marketing expenditure ought to be monitored closely.
If Avita executes its commercialisation strategy well and successfully attains FDA approval for its vitiligo trial, it may be a sound long-term investment.
If you’re looking for more places to park your capital, check out our ASX shares ideas hub where you’ll find lots of ASX stock ideas and analysis.










